mRNAs, proteins and the emerging principles of gene expression control
Gene expression involves transcription, translation and the turnover of mRNAs and proteins. The degree to which protein abundances scale with mRNA levels and the implications in cases where this dependency breaks down remain an intensely debated topic. Here we review recent mRNA–protein correlation studies in the light of the quantitative parameters of the gene expression pathway, contextual confounders and buffering mechanisms. Although protein and mRNA levels typically show reasonable correlation, we describe how transcriptomics and proteomics provide useful non-redundant readouts. Integrating both types of data can reveal exciting biology and is an essential step in refining our understanding of the principles of gene expression control.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
206,07 € per year
only 17,17 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
OmicScope unravels systems-level insights from quantitative proteomics data
Article Open access 02 August 2024
Quantification of translation uncovers the functions of the alternative transcriptome
Article 29 June 2020

grandR: a comprehensive package for nucleotide conversion RNA-seq data analysis
Article Open access 15 June 2023
References
- Abbott, S. & Fairbanks, D. J. Experiments on plant hybrids by Gregor Mendel. Genetics204, 407–422 (2016). PubMedPubMed CentralGoogle Scholar
- Lester, G. & Bonner, D. M. The occurrence of beta-galactosidase in Escherichia coli. J. Bacteriol.63, 759–769 (1952). CASPubMedPubMed CentralGoogle Scholar
- Jacob, F. & Monod, J. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol.3, 318–356 (1961). CASPubMedGoogle Scholar
- Gann, A. Jacob and Monod: from operons to EvoDevo. Curr. Biol.20, R718–R723 (2010). CASPubMedGoogle Scholar
- Montgomery, S. B. & Dermitzakis, E. T. From expression QTLs to personalized transcriptomics. Nat. Rev. Genet.12, 277–282 (2011). CASPubMedGoogle Scholar
- Koch, L. Genomics: adding another dimension to gene regulation. Nat. Rev. Genet.16, 563 (2015). CASPubMedGoogle Scholar
- Bentley, D. L. Coupling mRNA processing with transcription in time and space. Nat. Rev. Genet.15, 163–175 (2014). CASPubMedPubMed CentralGoogle Scholar
- Hentze, M. W., Castello, A., Schwarzl, T. & Preiss, T. A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol.19, 327–341 (2018). CASPubMedGoogle Scholar
- Tahmasebi, S., Khoutorsky, A., Mathews, M. B. & Sonenberg, N. Translation deregulation in human disease. Nat. Rev. Mol. Cell Biol.19, 791–807 (2018). CASPubMedGoogle Scholar
- Teixeira, F. K. & Lehmann, R. Translational control during developmental transitions. Cold Spring Harb. Perspect. Biol.11, a032987 (2019). CASPubMedPubMed CentralGoogle Scholar
- Emmott, E., Jovanovic, M. & Slavov, N. Ribosome stoichiometry: from form to function. Trends Biochem. Sci.44, 95–109 (2019). CASPubMedGoogle Scholar
- Schwartz, A. L. & Ciechanover, A. Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu. Rev. Pharmacol. Toxicol.49, 73–96 (2009). CASPubMedGoogle Scholar
- Pohl, C. & Dikic, I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science366, 818–822 (2019). CASPubMedGoogle Scholar
- Ryan, C. J. et al. High-resolution network biology: connecting sequence with function. Nat. Rev. Genet.14, 865–879 (2013). CASPubMedPubMed CentralGoogle Scholar
- Mann, M. & Jensen, O. N. Proteomic analysis of post-translational modifications. Nat. Biotechnol.21, 255–261 (2003). CASPubMedGoogle Scholar
- Alberts, B. et al. Molecular Biology of the Cell (Garland Press, 2002).
- Aebersold, R. et al. How many human proteoforms are there? Nat. Chem. Biol.14, 206–214 (2018). CASPubMedPubMed CentralGoogle Scholar
- Salovska, B. et al. Isoform-resolved correlation analysis between mRNA abundance regulation and protein level degradation. Mol. Syst. Biol.16, e9170 (2020). CASPubMedPubMed CentralGoogle Scholar
- Kempfer, R. & Pombo, A. Methods for mapping 3D chromosome architecture. Nat. Rev. Genet.21, 207–226 (2019). PubMedGoogle Scholar
- Goodwin, S., McPherson, J. D. & McCombie, W. R. Coming of age: ten years of next-generation sequencing technologies. Nat. Rev. Genet.17, 333–351 (2016). CASPubMedGoogle Scholar
- Stark, R., Grzelak, M. & Hadfield, J. RNA sequencing: the teenage years. Nat. Rev. Genet.20, 631–656 (2019). CASPubMedGoogle Scholar
- Aebersold, R. & Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature537, 347–355 (2016). This review provides an overview of mass spectrometry-based proteomic technologies and their biomedical applications. CASPubMedGoogle Scholar
- Vogel, C. & Marcotte, E. M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet.13, 227–232 (2012). CASPubMedPubMed CentralGoogle Scholar
- Liu, Y., Beyer, A. & Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell165, 535–550 (2016). CASPubMedGoogle Scholar
- Fortelny, N., Overall, C. M., Pavlidis, P. & Freue, G. V. C. Can we predict protein from mRNA levels? Nature547, E19–E20 (2017). This study questions the utility of protein-to-mRNA ratios and argues that these are likely to be of little use when one is attempting to make within-gene estimates of protein levels from mRNA. CASPubMedGoogle Scholar
- Franks, A., Airoldi, E. & Slavov, N. Post-transcriptional regulation across human tissues. PLoS Comput. Biol.13, e1005535 (2017). PubMedPubMed CentralGoogle Scholar
- Gygi, S. P., Rochon, Y., Franza, B. R. & Aebersold, R. Correlation between protein and mRNA abundance in yeast. Mol. Cell. Biol.19, 1720–1730 (1999). One of the original studies attempting to correlate proteins with mRNA abundance. Gygi and colleagues note that coverage bias may greatly affect across-gene correlations. CASPubMedPubMed CentralGoogle Scholar
- Edfors, F. et al. Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol. Syst. Biol.12, 883 (2016). PubMedPubMed CentralGoogle Scholar
- Zhang, B. et al. Proteogenomic characterization of human colon and rectal cancer. Nature513, 382–387 (2014). CASPubMedPubMed CentralGoogle Scholar
- Mertins, P. et al. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature534, 55–62 (2016). CASPubMedPubMed CentralGoogle Scholar
- Zhang, H. et al. Integrated proteogenomic characterization of human high-grade serous ovarian cancer. Cell166, 755–765 (2016). CASPubMedPubMed CentralGoogle Scholar
- Huang, K.-L. et al. Proteogenomic integration reveals therapeutic targets in breast cancer xenografts. Nat. Commun.8, 14864 (2017). PubMedPubMed CentralGoogle Scholar
- Archer, T. C. et al. Proteomics, post-translational modifications, and integrative analyses reveal molecular heterogeneity within medulloblastoma subgroups. Cancer Cell34, 396–410.e8 (2018). CASPubMedPubMed CentralGoogle Scholar
- Mun, D.-G. et al. Proteogenomic characterization of human early-onset gastric cancer. Cancer Cell35, 111–124.e10 (2019). CASPubMedGoogle Scholar
- Vasaikar, S. et al. Proteogenomic analysis of human colon cancer reveals new therapeutic opportunities. Cell177, 1035–1049.e19 (2019). CASPubMedPubMed CentralGoogle Scholar
- Khan, Z. et al. Primate transcript and protein expression levels evolve under compensatory selection pressures. Science342, 1100–1104 (2013). CASPubMedPubMed CentralGoogle Scholar
- Battle, A. et al. Genomic variation. Impact of regulatory variation from RNA to protein. Science347, 664–667 (2015). This study looks at how mRNA variation, arising from germline DNA variation in a population of humans, is buffered at the translational and protein levels. CASPubMedGoogle Scholar
- Chick, J. M. et al. Defining the consequences of genetic variation on a proteome-wide scale. Nature534, 500–505 (2016). CASPubMedPubMed CentralGoogle Scholar
- Wang, D. et al. A deep proteome and transcriptome abundance atlas of 29 healthy human tissues. Mol. Syst. Biol.15, e8503 (2019). PubMedPubMed CentralGoogle Scholar
- Ankney, J. A., Astor Ankney, J., Muneer, A. & Chen, X. Relative and absolute quantitation in mass spectrometry–based proteomics. Annu. Rev. Anal. Chem.11, 49–77 (2018). This review addresses the pros and cons of different absolute and relative proteomic quantification methods. CASGoogle Scholar
- Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods5, 621–628 (2008). CASPubMedGoogle Scholar
- Fu, Y., Wu, P.-H., Beane, T., Zamore, P. D. & Weng, Z. Elimination of PCR duplicates in RNA-seq and small RNA-seq using unique molecular identifiers. BMC Genomics19, 531 (2018). PubMedPubMed CentralGoogle Scholar
- Benjamini, Y. & Speed, T. P. Summarizing and correcting the GC content bias in high-throughput sequencing. Nucleic Acids Res.40, e72 (2012). CASPubMedPubMed CentralGoogle Scholar
- Tang, H. et al. A computational approach toward label-free protein quantification using predicted peptide detectability. Bioinformatics22, e481–e488 (2006). CASPubMedGoogle Scholar
- Zimmer, D., Schneider, K., Sommer, F., Schroda, M. & Mühlhaus, T. Artificial intelligence understands peptide observability and assists with absolute protein quantification. Front. Plant. Sci.9, 1559 (2018). PubMedPubMed CentralGoogle Scholar
- Peng, M. et al. Protease bias in absolute protein quantitation. Nat. Methods9, 524–525 (2012). This study specifically interrogates intensity-based quantification methods in mass-spectrometry-based proteomics and how these may differ widely depending on the mode of enzyme digestion. CASPubMedGoogle Scholar
- Schwanhäusser, B. et al. Global quantification of mammalian gene expression control. Nature473, 337–342 (2011). This study combines metabolic pulse labelling and absolute quantification of both mRNAs and proteins with mathematical modelling to quantify the major stages of mammalian gene expression control. PubMedGoogle Scholar
- Li, J. J., Bickel, P. J. & Biggin, M. D. System wide analyses have underestimated protein abundances and the importance of transcription in mammals. PeerJ2, e270 (2014). PubMedPubMed CentralGoogle Scholar
- Jovanovic, M. et al. Dynamic profiling of the protein life cycle in response to pathogens. Science347, 1259038 (2015). This study integrates absolute quantification of mRNAs and proteins along with protein turnover information in the context of LPS stimulation using ordinary differential equations to comprehensively assess gene expression regulation. PubMedPubMed CentralGoogle Scholar
- Ahrné, E., Molzahn, L., Glatter, T. & Schmidt, A. Critical assessment of proteome-wide label-free absolute abundance estimation strategies. Proteomics13, 2567–2578 (2013). PubMedGoogle Scholar
- Zeiler, M., Straube, W. L., Lundberg, E., Uhlen, M. & Mann, M. A protein epitope signature tag (PrEST) library allows SILAC-based absolute quantification and multiplexed determination of protein copy numbers in cell lines. Mol. Cell. Proteom.11, O111.009613 (2012). Google Scholar
- Lin, C. Y. et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell151, 56–67 (2012). CASPubMedPubMed CentralGoogle Scholar
- Crick, F. Central dogma of molecular biology. Nature227, 561–563 (1970). This study provides the original postulate of the central dogma of molecular biology, not to be confused with gene expression in general. CASPubMedGoogle Scholar
- Cobb, M. 60 years ago, Francis Crick changed the logic of biology. PLoS Biol.15, e2003243 (2017). PubMedPubMed CentralGoogle Scholar
- Davidson, E. H. Emerging properties of animal gene regulatory networks. Nature468, 911–920 (2010). CASPubMedPubMed CentralGoogle Scholar
- Lindeboom, R. G. H. et al. Integrative multi-omics analysis of intestinal organoid differentiation. Mol. Syst. Biol.14, e8227 (2018). PubMedPubMed CentralGoogle Scholar
- Becker, K. et al. Quantifying post-transcriptional regulation in the development of Drosophila melanogaster. Nat. Commun.9, 4970 (2018). PubMedPubMed CentralGoogle Scholar
- Ingolia, N. T., Lareau, L. F. & Weissman, J. S. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell147, 789–802 (2011). CASPubMedPubMed CentralGoogle Scholar
- Cheng, Z. et al. Differential dynamics of the mammalian mRNA and protein expression response to misfolding stress. Mol. Syst. Biol.12, 855 (2016). PubMedPubMed CentralGoogle Scholar
- Darzacq, X. et al. In vivo dynamics of RNA polymerase II transcription. Nat. Struct. Mol. Biol.14, 796–806 (2007). CASPubMedPubMed CentralGoogle Scholar
- Hausser, J., Mayo, A., Keren, L. & Alon, U. Central dogma rates and the trade-off between precision and economy in gene expression. Nat. Commun.10, 68 (2019). This study analyses the parametric landscape of gene expression across genes, investigating the overall strategy evolution has selected, for example, to regulate highly expressed genes. CASPubMedPubMed CentralGoogle Scholar
- Schwanhäusser, B., Wolf, J., Selbach, M. & Busse, D. Synthesis and degradation jointly determine the responsiveness of the cellular proteome. Bioessays35, 597–601 (2013). PubMedGoogle Scholar
- Beyer, A., Hollunder, J., Nasheuer, H.-P. & Wilhelm, T. Post-transcriptional expression regulation in the yeast Saccharomyces cerevisiae on a genomic scale. Mol. Cell. Proteomics3, 1083–1092 (2004). CASPubMedGoogle Scholar
- Zecha, J. et al. Peptide level turnover measurements enable the study of proteoform dynamics. Mol. Cell. Proteomics17, 974–992 (2018). CASPubMedPubMed CentralGoogle Scholar
- Kristensen, A. R., Gsponer, J. & Foster, L. J. Protein synthesis rate is the predominant regulator of protein expression during differentiation. Mol. Syst. Biol.9, 689 (2013). PubMedPubMed CentralGoogle Scholar
- Baum, K., Schuchhardt, J., Wolf, J. & Busse, D. Of gene expression and cell division time: a mathematical framework for advanced differential gene expression and data analysis. Cell Syst.9, 569–579 (2019). This study formalizes the role of cell cycle time in the context of gene expression. CASPubMedGoogle Scholar
- Wilhelm, M. et al. Mass-spectrometry-based draft of the human proteome. Nature509, 582–587 (2014). This study presents an in-depth proteomics and transcriptomics dataset of 12 tissues and is one of the first to posit that protein-to-mRNA ratios can be used to estimate absolute protein abundances from mRNA. CASPubMedGoogle Scholar
- Eraslan, B. et al. Quantification and discovery of sequence determinants of protein-per-mRNA amount in 29 human tissues. Mol. Syst. Biol.15, e8513 (2019). PubMedPubMed CentralGoogle Scholar
- Buszczak, M., Signer, R. A. J. & Morrison, S. J. Cellular differences in protein synthesis regulate tissue homeostasis. Cell159, 242–251 (2014). CASPubMedPubMed CentralGoogle Scholar
- Peshkin, L. et al. On the relationship of protein and mRNA dynamics in vertebrate embryonic development. Dev. Cell35, 383–394 (2015). CASPubMedPubMed CentralGoogle Scholar
- Xiao, H. et al. Differential proteomic analysis of human saliva using tandem mass tags quantification for gastric cancer detection. Sci. Rep.6, 22165 (2016). CASPubMedPubMed CentralGoogle Scholar
- Zhao, M. et al. A comprehensive analysis and annotation of human normal urinary proteome. Sci. Rep.7, 3024 (2017). PubMedPubMed CentralGoogle Scholar
- Cso˝sz, É. et al. Quantitative body fluid proteomics in medicine - a focus on minimal invasiveness. J. Proteomics153, 30–43 (2017). PubMedGoogle Scholar
- Geyer, P. E., Holdt, L. M., Teupser, D. & Mann, M. Revisiting biomarker discovery by plasma proteomics. Mol. Syst. Biol.13, 942 (2017). PubMedPubMed CentralGoogle Scholar
- Yao, C. et al. Genome-wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat. Commun.9, 3268 (2018). PubMedPubMed CentralGoogle Scholar
- Meissner, F., Scheltema, R. A., Mollenkopf, H.-J. & Mann, M. Direct proteomic quantification of the secretome of activated immune cells. Science340, 475–478 (2013). CASPubMedGoogle Scholar
- Suhre, K. et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun.8, 14357 (2017). CASPubMedPubMed CentralGoogle Scholar
- Moritz, C. P., Mühlhaus, T., Tenzer, S., Schulenborg, T. & Friauf, E. Poor transcript-protein correlation in the brain: negatively correlating gene products reveal neuronal polarity as a potential cause. J. Neurochem.149, 582–604 (2019). CASPubMedGoogle Scholar
- Zappulo, A. et al. RNA localization is a key determinant of neurite-enriched proteome. Nat. Commun.8, 583 (2017). PubMedPubMed CentralGoogle Scholar
- Holt, C. E., Martin, K. C. & Schuman, E. M. Local translation in neurons: visualization and function. Nat. Struct. Mol. Biol.26, 557–566 (2019). CASPubMedGoogle Scholar
- Chekulaeva, M. & Landthaler, M. Eyes on translation. Mol. Cell63, 918–925 (2016). CASPubMedGoogle Scholar
- Sysoev, V. O. et al. Global changes of the RNA-bound proteome during the maternal-to-zygotic transition in Drosophila. Nat. Commun.7, 12128 (2016). CASPubMedPubMed CentralGoogle Scholar
- Stoeckius, M. et al. Global characterization of the oocyte-to-embryo transition in Caenorhabditis elegans uncovers a novel mRNA clearance mechanism. EMBO J.33, 1751–1766 (2014). CASPubMedPubMed CentralGoogle Scholar
- Koch, S., Acebron, S. P., Herbst, J., Hatiboglu, G. & Niehrs, C. Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell163, 1225–1236 (2015). CASPubMedGoogle Scholar
- Liu, X. et al. Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation. Nat. Cell Biol.19, 626–638 (2017). CASPubMedPubMed CentralGoogle Scholar
- Gautier, E.-F. et al. Comprehensive proteomic analysis of human erythropoiesis. Cell Rep.16, 1470–1484 (2016). CASPubMedPubMed CentralGoogle Scholar
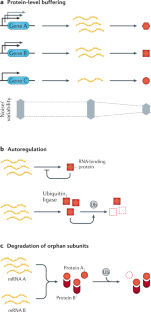
- Nguyen, A. T. et al. UBE2O remodels the proteome during terminal erythroid differentiation. Science357, eaan0218 (2017). PubMedPubMed CentralGoogle Scholar
- Yanagitani, K., Juszkiewicz, S. & Hegde, R. S. UBE2O is a quality control factor for orphans of multiprotein complexes. Science357, 472–475 (2017). Nguyen et al. (2017) and Yanagitani et al. (2017) characterize the mechanism of action and role of UBE2O as a ubiquitin ligase responsible for clearing surplus protein complex subunits. CASPubMedPubMed CentralGoogle Scholar
- Mathieson, T. et al. Systematic analysis of protein turnover in primary cells. Nat. Commun.9, 689 (2018). PubMedPubMed CentralGoogle Scholar
- Dörrbaum, A. R., Kochen, L., Langer, J. D. & Schuman, E. M. Local and global influences on protein turnover in neurons and glia. eLife7, e34202 (2018). PubMedPubMed CentralGoogle Scholar
- Marguerat, S. et al. Quantitative analysis of fission yeast transcriptomes and proteomes in proliferating and quiescent cells. Cell151, 671–683 (2012). CASPubMedPubMed CentralGoogle Scholar
- Félix, M.-A. & Barkoulas, M. Pervasive robustness in biological systems. Nat. Rev. Genet.16, 483–496 (2015). PubMedGoogle Scholar
- Rogers, J. & Gibbs, R. A. Comparative primate genomics: emerging patterns of genome content and dynamics. Nat. Rev. Genet.15, 347–359 (2014). CASPubMedPubMed CentralGoogle Scholar
- Waszak, S. M. et al. Population variation and genetic control of modular chromatin architecture in humans. Cell162, 1039–1050 (2015). CASPubMedGoogle Scholar
- Raj, A., Peskin, C. S., Tranchina, D., Vargas, D. Y. & Tyagi, S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol.4, e309 (2006). PubMedPubMed CentralGoogle Scholar
- Raser, J. M. & O’Shea, E. K. Control of stochasticity in eukaryotic gene expression. Science304, 1811–1814 (2004). CASPubMedPubMed CentralGoogle Scholar
- Kustatscher, G., Grabowski, P. & Rappsilber, J. Pervasive coexpression of spatially proximal genes is buffered at the protein level. Mol. Syst. Biol.13, 937 (2017). This study outlines how covariation in mRNA owing to chromosomal location of source genes is lost at the protein level. PubMedPubMed CentralGoogle Scholar
- Geiger, T., Cox, J. & Mann, M. Proteomic changes resulting from gene copy number variations in cancer cells. PLoS Genet.6, e1001090 (2010). This is the first systematic analysis showing that the levels of some proteins are resistant to DNA copy number changes in mammalian cell lines. PubMedPubMed CentralGoogle Scholar
- Stingele, S. et al. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol. Syst. Biol.8, 608 (2012). PubMedPubMed CentralGoogle Scholar
- Dephoure, N. et al. Quantitative proteomic analysis reveals posttranslational responses to aneuploidy in yeast. eLife3, e03023 (2014). PubMedPubMed CentralGoogle Scholar
- Gonçalves, E. et al. Widespread post-transcriptional attenuation of genomic copy-number variation in cancer. Cell Syst.5, 386–398.e4 (2017). This study presents a reanalysis of Clinical Proteomic Tumor Analysis Consortium (CPTAC) breast, ovarian and colorectal cancer studies and finds that ~20–30% of mRNA changes caused by aneuploidy are buffered at the protein level and further leverage this information to predict protein–protein interactions. PubMedPubMed CentralGoogle Scholar
- Liu, Y. et al. Systematic proteome and proteostasis profiling in human Trisomy 21 fibroblast cells. Nat. Commun.8, 1212 (2017). PubMedPubMed CentralGoogle Scholar
- Schlattl, A., Anders, S., Waszak, S. M., Huber, W. & Korbel, J. O. Relating CNVs to transcriptome data at fine resolution: assessment of the effect of variant size, type, and overlap with functional regions. Genome Res.21, 2004–2013 (2011). CASPubMedPubMed CentralGoogle Scholar
- Fehrmann, R. S. N. et al. Gene expression analysis identifies global gene dosage sensitivity in cancer. Nat. Genet.47, 115–125 (2015). This study demonstrates, across tens of thousands of microarray and RNA-seq samples, the direct and dosage-sensitive effects of somatic copy number alterations (aneuploidy) on mRNA and underlines almost no buffering between the DNA and mRNA levels. CASPubMedGoogle Scholar
- Grönlund, A., Lötstedt, P. & Elf, J. Transcription factor binding kinetics constrain noise suppression via negative feedback. Nat. Commun.4, 1864 (2013). PubMedGoogle Scholar
- Müller-McNicoll, M., Rossbach, O., Hui, J. & Medenbach, J. Auto-regulatory feedback by RNA-binding proteins. J. Mol. Cell Biol.11, 930–939 (2019). PubMedPubMed CentralGoogle Scholar
- Jumaa, H. & Nielsen, P. J. The splicing factor SRp20 modifies splicing of its own mRNA and ASF/SF2 antagonizes this regulation. EMBO J.16, 5077–5085 (1997). This is one of the early studies analysing the autoregulatory capability of many splicing factors. CASPubMedPubMed CentralGoogle Scholar
- Lareau, L. F., Inada, M., Green, R. E., Wengrod, J. C. & Brenner, S. E. Unproductive splicing of SR genes associated with highly conserved and ultraconserved DNA elements. Nature446, 926–929 (2007). CASPubMedGoogle Scholar
- de Bie, P. & Ciechanover, A. Ubiquitination of E3 ligases: self-regulation of the ubiquitin system via proteolytic and non-proteolytic mechanisms. Cell Death Differ.18, 1393–1402 (2011). PubMedPubMed CentralGoogle Scholar
- Signor, S. A. & Nuzhdin, S. V. The evolution of gene expression in cis and trans. Trends Genet.34, 532–544 (2018). CASPubMedPubMed CentralGoogle Scholar
- Bader, D. M. et al. Negative feedback buffers effects of regulatory variants. Mol. Syst. Biol.11, 785 (2015). PubMedPubMed CentralGoogle Scholar
- Battich, N., Stoeger, T. & Pelkmans, L. Control of transcript variability in single mammalian cells. Cell163, 1596–1610 (2015). CASPubMedGoogle Scholar
- Artieri, C. G. & Fraser, H. B. Evolution at two levels of gene expression in yeast. Genome Res.24, 411–421 (2014). CASPubMedPubMed CentralGoogle Scholar
- McManus, C. J., May, G. E., Spealman, P. & Shteyman, A. Ribosome profiling reveals post-transcriptional buffering of divergent gene expression in yeast. Genome Res.24, 422–430 (2014). CASPubMedPubMed CentralGoogle Scholar
- Taggart, J. C. & Li, G.-W. Production of protein-complex components is stoichiometric and lacks general feedback regulation in eukaryotes. Cell Syst.7, 580–589.e4 (2018). CASPubMedPubMed CentralGoogle Scholar
- Juszkiewicz, S. & Hegde, R. S. Quality control of orphaned proteins. Mol. Cell71, 443–457 (2018). CASPubMedPubMed CentralGoogle Scholar
- McShane, E. et al. Kinetic analysis of protein stability reveals age-dependent degradation. Cell167, 803–815.e21 (2016). CASPubMedGoogle Scholar
- Taggart, J. C., Zauber, H., Selbach, M., Li, G.-W. & McShane, E. Keeping the proportions of protein complex components in check. Cell Syst.10, 125–132 (2020). CASPubMedPubMed CentralGoogle Scholar
- Santaguida, S., Vasile, E., White, E. & Amon, A. Aneuploidy-induced cellular stresses limit autophagic degradation. Genes Dev.29, 2010–2021 (2015). CASPubMedPubMed CentralGoogle Scholar
- Santaguida, S. et al. Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the immune system. Dev. Cell41, 638–651.e5 (2017). CASPubMedPubMed CentralGoogle Scholar
- Stuart, T. & Satija, R. Integrative single-cell analysis. Nat. Rev. Genet.20, 257–272 (2019). CASPubMedGoogle Scholar
- Marx, V. A dream of single-cell proteomics. Nat. Methods16, 809–812 (2019). CASPubMedGoogle Scholar
- Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods14, 865–868 (2017). CASPubMedPubMed CentralGoogle Scholar
- Spitzer, M. H. & Nolan, G. P. Mass cytometry: single cells, many features. Cell165, 780–791 (2016). CASPubMedPubMed CentralGoogle Scholar
- Budnik, B., Levy, E., Harmange, G. & Slavov, N. SCoPE-MS: mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biol.19, 161 (2018). PubMedPubMed CentralGoogle Scholar
- Popovic, D., Koch, B., Kueblbeck, M., Ellenberg, J. & Pelkmans, L. Multivariate control of transcript to protein variability in single mammalian cells. Cell Syst.7, 398–411.e6 (2018). CASPubMedGoogle Scholar
- Albayrak, C. et al. Digital quantification of proteins and mRNA in single mammalian cells. Mol. Cell61, 914–924 (2016). CASPubMedGoogle Scholar
- Genshaf, A. S. et al. Multiplexed, targeted profiling of single-cell proteomes and transcriptomes in a single reaction. Genome Biol.17, 188 (2016). Google Scholar
- Peterson, V. M. et al. Multiplexed quantification of proteins and transcripts in single cells. Nat. Biotechnol.35, 936–939 (2017). CASPubMedGoogle Scholar
- Sopko, R. et al. Mapping pathways and phenotypes by systematic gene overexpression. Mol. Cell21, 319–330 (2006). CASPubMedGoogle Scholar
- Brennan, C. M. et al. Protein aggregation mediates stoichiometry of protein complexes in aneuploid cells. Genes Dev.33, 1031–1047 (2019). CASPubMedPubMed CentralGoogle Scholar
- Klosin, A. et al. Phase separation provides a mechanism to reduce noise in cells. Science367, 464–468 (2020). CASPubMedGoogle Scholar
- Nusinow, D. P. et al. Quantitative proteomics of the cancer cell line encyclopedia. Cell180, 387–402.e16 (2020). CASPubMedPubMed CentralGoogle Scholar
- Carter, S. L., Eklund, A. C., Kohane, I. S., Harris, L. N. & Szallasi, Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat. Genet.38, 1043–1048 (2006). CASPubMedGoogle Scholar
- Ben-David, U. et al. The landscape of chromosomal aberrations in breast cancer mouse models reveals driver-specific routes to tumorigenesis. Nat. Commun.7, 12160 (2016). CASPubMedPubMed CentralGoogle Scholar
- Gehre, M., Buccitelli, C., Diaz, N., Korbel, J. & Noh, K.-M. Efficient strategies to detect genome editing and integrity in CRISPR-Cas9 engineered ESCs. Preprint at bioRxivhttps://doi.org/10.1101/635151 (2019).
- Wang, J. et al. Proteome profiling outperforms transcriptome profiling for coexpression based gene function prediction. Mol. Cell. Proteom.16, 121–134 (2017). CASGoogle Scholar
- Lapek, J. D. Jr et al. Detection of dysregulated protein-association networks by high-throughput proteomics predicts cancer vulnerabilities. Nat. Biotechnol.35, 983–989 (2017). CASPubMedPubMed CentralGoogle Scholar
- Kustatscher, G. et al. Co-regulation map of the human proteome enables identification of protein functions. Nat. Biotechnol.37, 1361–1371 (2019). CASPubMedPubMed CentralGoogle Scholar
- Roumeliotis, T. I. et al. Genomic determinants of protein abundance variation in colorectal cancer cells. Cell Rep.20, 2201–2214 (2017). CASPubMedPubMed CentralGoogle Scholar
- Romanov, N. et al. Disentangling genetic and environmental effects on the proteotypes of individuals. Cell177, 1308–1318.e10 (2019). CASPubMedPubMed CentralGoogle Scholar
- Ori, A. et al. Spatiotemporal variation of mammalian protein complex stoichiometries. Genome Biol.17, 47 (2016). PubMedPubMed CentralGoogle Scholar
- Doll, S., Gnad, F. & Mann, M. The case for proteomics and phospho-proteomics in personalized cancer medicine. Proteomics Clin. Appl.13, e1800113 (2019). PubMedGoogle Scholar
- Welker, F. et al. Enamel proteome shows that Gigantopithecus was an early diverging pongine. Nature576, 262–265 (2019). CASPubMedPubMed CentralGoogle Scholar
- Olsen, J. V. et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell127, 635–648 (2006). CASPubMedGoogle Scholar
- Meyer, K. & Selbach, M. Quantitative affinity purification mass spectrometry: a versatile technology to study protein-protein interactions. Front. Genet.6, 237 (2015). PubMedPubMed CentralGoogle Scholar
- Yang, J., Wagner, S. A. & Beli, P. Illuminating spatial and temporal organization of protein interaction networks by mass spectrometry-based proteomics. Front. Genet.6, 344 (2015). PubMedPubMed CentralGoogle Scholar
- Dettmer, K., Aronov, P. A. & Hammock, B. D. Mass spectrometry-based metabolomics. Mass. Spectrom. Rev.26, 51–78 (2007). CASPubMedPubMed CentralGoogle Scholar
- Zelezniak, A. et al. Machine learning predicts the yeast metabolome from the quantitative proteome of kinase knockouts. Cell Syst.7, 269–283.e6 (2018). CASPubMedPubMed CentralGoogle Scholar
- Senft, D., Qi, J. & Ronai, Z. A. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat. Rev. Cancer18, 69–88 (2017). PubMedPubMed CentralGoogle Scholar
- Krönke, J. et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science343, 301–305 (2014). PubMedGoogle Scholar
- Lu, G. et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science343, 305–309 (2014). CASPubMedGoogle Scholar
- Chamberlain, P. P. & Hamann, L. G. Development of targeted protein degradation therapeutics. Nat. Chem. Biol.15, 937–944 (2019). CASPubMedGoogle Scholar
- Tang, Y.-C., Williams, B. R., Siegel, J. J. & Amon, A. Identification of aneuploidy-selective antiproliferation compounds. Cell144, 499–512 (2011). CASPubMedPubMed CentralGoogle Scholar
- Ben-David, U. & Amon, A. Context is everything: aneuploidy in cancer. Nat. Rev. Genet.21, 44–62 (2020). CASPubMedGoogle Scholar
- Hasin, Y., Seldin, M. & Lusis, A. Multi-omics approaches to disease. Genome Biol.18, 83 (2017). PubMedPubMed CentralGoogle Scholar
- Vitrinel, B. et al. Exploiting interdata relationships in next-generation proteomics analysis. Mol. Cell. Proteomics18, S5–S14 (2019). CASPubMedPubMed CentralGoogle Scholar
- Dölken, L. et al. High-resolution gene expression profiling for simultaneous kinetic parameter analysis of RNA synthesis and decay. RNA14, 1959–1972 (2008). PubMedPubMed CentralGoogle Scholar
- Herzog, V. A. et al. Thiol-linked alkylation of RNA to assess expression dynamics. Nat. Methods14, 1198–1204 (2017). CASPubMedPubMed CentralGoogle Scholar
- Mann, M. Functional and quantitative proteomics using SILAC. Nat. Rev. Mol. Cell Biol.7, 952–958 (2006). CASPubMedGoogle Scholar
- Selbach, M. et al. Widespread changes in protein synthesis induced by microRNAs. Nature455, 58–63 (2008). CASPubMedGoogle Scholar
- Schwanhäusser, B., Gossen, M., Dittmar, G. & Selbach, M. Global analysis of cellular protein translation by pulsed SILAC. Proteomics9, 205–209 (2009). PubMedGoogle Scholar
- Doherty, M. K., Hammond, D. E., Clague, M. J., Gaskell, S. J. & Beynon, R. J. Turnover of the human proteome: determination of protein intracellular stability by dynamic SILAC. J. Proteome Res.8, 104–112 (2009). CASPubMedGoogle Scholar
- Boisvert, F.-M. et al. A quantitative spatial proteomics analysis of proteome turnover in human cells. Mol. Cell. Proteomics11, M111.011429 (2012). PubMedGoogle Scholar
- Savitski, M. M. et al. Multiplexed proteome dynamics profiling reveals mechanisms controlling protein homeostasis. Cell173, 260–274.e25 (2018). CASPubMedPubMed CentralGoogle Scholar
- Dieterich, D. C., Link, A. J., Graumann, J., Tirrell, D. A. & Schuman, E. M. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT). Proc. Natl Acad. Sci. USA103, 9482–9487 (2006). CASPubMedPubMed CentralGoogle Scholar
- Eichelbaum, K., Winter, M., Berriel Diaz, M., Herzig, S. & Krijgsveld, J. Selective enrichment of newly synthesized proteins for quantitative secretome analysis. Nat. Biotechnol.30, 984–990 (2012). CASPubMedGoogle Scholar
- Brar, G. A. & Weissman, J. S. Ribosome profiling reveals the what, when, where and how of protein synthesis. Nat. Rev. Mol. Cell Biol.16, 651–664 (2015). CASPubMedPubMed CentralGoogle Scholar
- van Heesch, S. et al. The translational landscape of the human heart. Cell178, 242–260.e29 (2019). PubMedGoogle Scholar
- Calviello, L. et al. Detecting actively translated open reading frames in ribosome profiling data. Nat. Methods13, 165–170 (2016). CASPubMedGoogle Scholar
- Diament, A. et al. The extent of ribosome queuing in budding yeast. PLoS Comput. Biol.14, e1005951 (2018). PubMedPubMed CentralGoogle Scholar
- Riba, A. et al. Protein synthesis rates and ribosome occupancies reveal determinants of translation elongation rates. Proc. Natl Acad. Sci. USA116, 15023–15032 (2019). CASPubMedPubMed CentralGoogle Scholar
- Duncan, C. D. S. & Mata, J. Effects of cycloheximide on the interpretation of ribosome profiling experiments in Schizosaccharomyces pombe. Sci. Rep.7, 10331 (2017). PubMedPubMed CentralGoogle Scholar
- Ingolia, N. T. Tracking the missing footprints of idle ribosomes. Cell Syst.4, 583–584 (2017). CASPubMedGoogle Scholar
- Liu, T.-Y. et al. Time-resolved proteomics extends ribosome profiling-based measurements of protein synthesis dynamics. Cell Syst.4, 636–644.e9 (2017). CASPubMedPubMed CentralGoogle Scholar
- Anscombe, F. J. Graphs in statistical analysis. Am. Stat.27, 17–21 (1973). Google Scholar
Acknowledgements
The authors acknowledge the extensive contributions of the scientific community to the topic of this Review and apologize for being unable to reference all pertinent articles. The authors thank P. Mertins, J. Wolf, D. Harnett (all from the Max Delbrück Center for Molecular Medicine) and E. McShane (Harvard Medical School) for feedback. They also thank T. Melder (Max Delbrück Center for Molecular Medicine) for help with the chemical structures and all other members of the Selbach laboratory for helpful discussions.










